Summary: In this Article, you’ll get to read about —
Metals play a key role in our everyday lives, from the construction of buildings to the manufacturing of electronic devices. One fundamental property that distinguishes alloys is their fusion point – the temperature at which they transition from a solid to a liquid state.
A metal’s melting point can be described as a moment it transitions itself from solid to liquid phase. When a metal achieves its fusion temperature its solid and liquid phases coexist and once it passes it becomes liquid. Thus, understanding the metal points is vital in choosing a suitable metal for your project.
In this blog, we will delve into the fascinating world of metal fusion temperature, exploring the factors that influence them and highlighting the significance of one particular alloy: aluminum.
I. Introduction
Alloys form a vital category of elements characterized by their luster, conductivity, and malleability. One key aspect that sets them apart is their varying flash points. Understanding these melting positions is vital for various applications, from metallurgy to the design of high-temperature materials.
II. What Influences Melting Points?
Several factors contribute to the fusion points of alloys, including atomic structure, bonding type, and intermolecular forces. The arrangement of atoms in an alloy’s crystal lattice and the strength of the metallic bonds significantly impact its response to heat.
III. The Range of Melting Points
Metals exhibit a broad range of melting points. Some, like mercury, have low flash positions and are liquid at room temperature, while others, such as tungsten, boast exceptionally high flash positions, making them suitable for applications in extreme environments.
Most Abundant Metal on Earth?
Iron is the most abundant metal on earth. However, the most common metal found on Earth’s crust is aluminum.
IV. Aluminum: A Highlight
Aluminum is a post-transition metal in the boron group and has a density lower than other common metals. It has a lower melting temperature compared to other metals like iron, brass, and copper, which is approx 660 degrees Celsius or 1200 degrees Fahrenheit.
A. Introduction to Aluminum
Aluminum is the most abundant metal in the Earth’s crust, which is approximately 8.1%, but rarely found uncombined. It has a symbol of AI and an atomic number of 13. visually it resembles silver bot in color and its ability to reflect light, it has a great affinity towards oxygen and when exposed to aid it forms a protective layer of oxide on the surface.
It is a lightweight and versatile metal and holds a special place in various industries. Its applications range from aerospace engineering to packaging materials.
B. The Melting Point of Aluminum
Among the myriad of metals, it stands out with its moderate point. At approximately 660.32 ° C (1220.58 °F), it undergoes the phase transition from solid to liquid. Do you want to know more about the melting positions of aluminum? Click here.
C. Significance in Industrial Processes
Its relatively low melting point makes it easily workable in industrial processes such as casting and extrusion. Its formability at lower temperatures contributes to the efficiency of manufacturing processes.
V. Practical Implications
Understanding the fusion points of metals has profound implications for designing materials for specific uses. Engineers and scientists leverage this knowledge to select appropriate materials for diverse applications, ensuring optimal performance under varying conditions.
Now, let’s take a brief look at the fusion positions of some common alloys:
Iron – Melting Point: 1538 °c(2800 °F)
Copper- flash positions: 1084 °c (1983 °F)
Gold- fusion positions: 1064 °c (1947 °F)
Silver – Melting Point: 961 °c(1761 °F)
Lead – flash positions: 327 °c (621 °F)
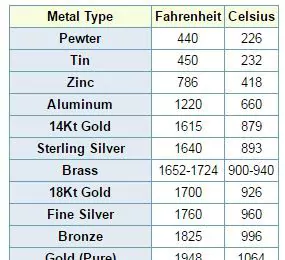
These values represent just a small fraction of the vast array of metals and their respective melting points. The ability to manipulate these temperatures has been instrumental in shaping the modern world, from the construction of buildings to the development of advanced technologies.
Did You Know?
Tungsten has the highest melting point of any metal, which is 3,400 °c. Although Carbon remains solid at higher temperatures it transitions into gas rather than melting into liquid.
VI. Conclusion
In conclusion, the melting points of metals play a pivotal role in shaping the materials we use daily. From the lowest to the highest flash positions, each alloy’s unique properties contribute to its applications in different industries. Aluminum, with its moderate flash positions, exemplifies the versatility of alloys and their impact on our technological advancements.
As we continue to explore the properties of metals, we gain insights that pave the way for innovations and improvements in various fields. In case it’s the malleability of aluminum or the high fusion points of refractory ore, each aspect contributes to the intricate tapestry of materials science.
HXSCO is a leading alloy supplier. If you want to buy ore products or want to know basic information about alloys, please contact this professional metal supplier.